AVS Raises $8.8M in Series B Funding to Accelerate Clinical Trials for Pulsatile Intravascular Lithotripsy Solutions.
April 6, 2023
AVS announced $8.8 million Series B financing round. The early-stage medical device company's primary goal is to treat severely calcified arterial disease. The Series B financing round was led by BioStar Capital and CUE Growth Partners.
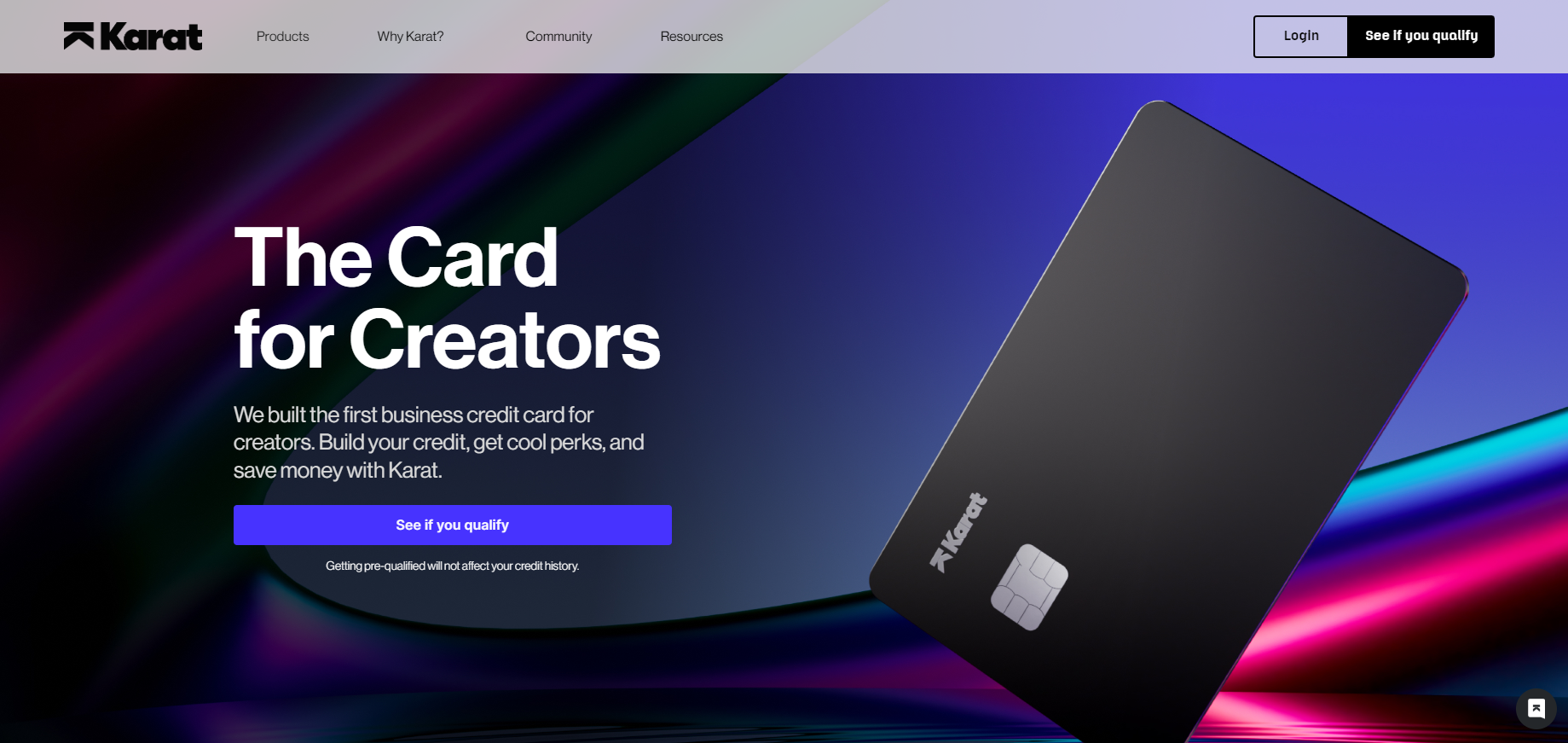
AVS is an early-stage medical device company focused on treating severely calcified arterial disease in a safe and effective manner. Hitinder Gurm, M.D., Interventional Cardiologist and Chief Clinical Officer at the University of Michigan, and Robert Chisena, Ph.D., Chief Technical Officer at AVS founded it in 2018.
The new funding, according to AVS, will accelerate clinical trial timelines for the company's Pulse device, a pulsatile intravascular lithotripsy (IVL) system for peripheral applications, as well as advance development and preclinical work on a pulsatile IVL device for coronary cases. The Pulse system is a balloon-based platform that uses pressure waves in frequent bursts to break up calcium and expand calcified arteries.
"Our preliminary trial results demonstrated that we can successfully treat patients with multiple lesions with a single device," Dr. Jon George, MD, an interventional cardiologist at the University of Pennsylvania Health System in Philadelphia, Pennsylvania, said in an AVS press release. "In our initial study, we saw patients report a reduction in leg pain, an increase in blood flow to the leg, and an improvement in their ability to walk." This patient population requires easier access to advanced therapies, which this platform has the potential to provide."